STEADFAST
A CLINICAL RESEARCH STUDY
INVESTIGATING GENETICALLY MODIFIED CELL THERAPY TX200
FOR PATIENTS UNDERGOING A KIDNEY TRANSPLANT
ABOUT ESRD AND KIDNEY TRANSPLANT
End-stage renal disease (ESRD) is the last stage of chronic kidney disease, when a person’s kidneys are no longer working. A kidney transplant is considered the best treatment option for ESRD. A kidney transplant cannot take place without the generosity of an organ donor, who can be either living or deceased.
Following kidney transplant surgery, the recipient needs to take lifelong immunosuppressive medications to suppress their immune system and help prevent their body from rejecting the transplanted kidney. These medications often do not target the transplanted kidney itself, but act to suppress the body’s entire immune system. Consequently, immunosuppressive medications can be associated with side effects such as an increased risk of infections or other serious conditions.
In addition, kidney transplant recipients are required to take these immunosuppressive medications for the life of the transplanted kidney. Therefore, there is a need to find an alternative approach to help the body accept the donor organ and reduce the need for immunosuppressive medication.


iA Multicentre Single Ascending Dose, Open-label Phase I/IIa study to evaluate the safety and tolerability of an autologous Antigen-Specific Chimeric Antigen Receptor T regulatory cell therapy in living donor renal transplant recipients.
WHAT IS TX200?
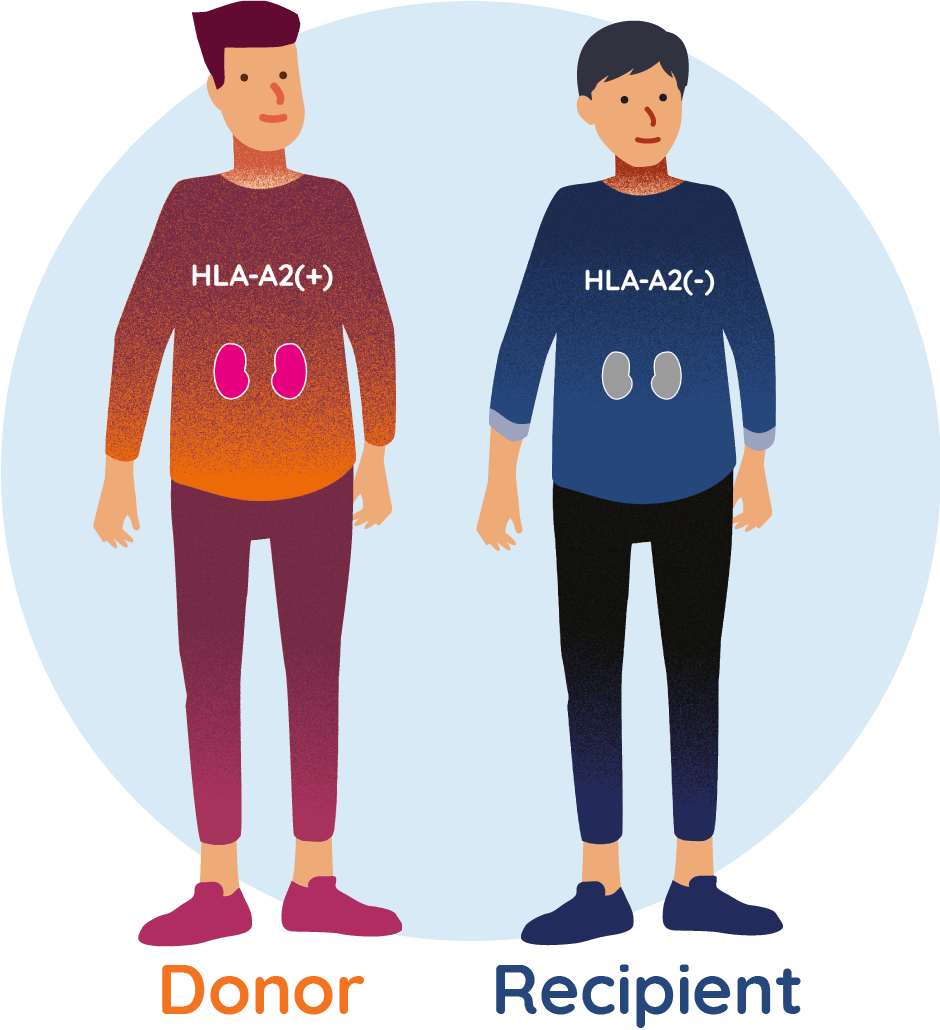
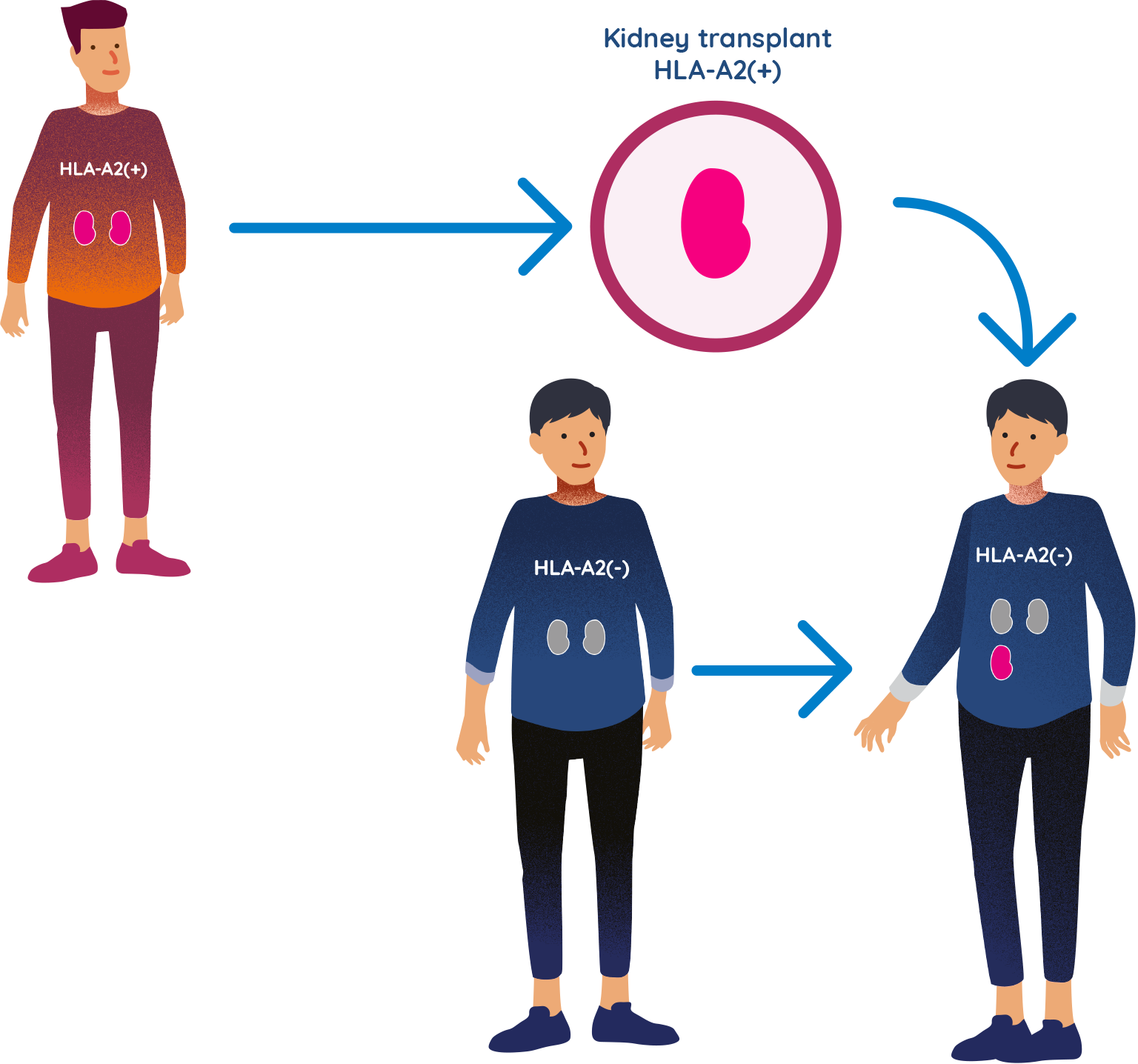
TX200 is an investigational genetically modified cell therapy developed for those receiving a kidney transplant from a living donor, where the recipient of the kidney is HLA-A2 negative and the donor is HLA-A2 positive. Overall, it is estimated that this ‘mismatch’ occurs in approximately 20–25% of all organ transplantations.
It is hoped that TX200 could potentially encourage acceptance of the new kidney by the recipient’s immune system, therefore reducing the need for immunosuppressive medication.
WHAT IS A PHASE I/II CLINICAL STUDY?
An investigational medicine or study drug is first examined in a small number of people in Phase I/II clinical studies to evaluate its safety and side effects. Rigorous scientific testing is done before this step.
Once the safety of the study drug is better understood, it can be tested further in more patients to continue to monitor safety as well as to examine effectiveness. All medicines have to go through these very rigorous steps before they can receive a marketing authorisation and be prescribed to people.
WHAT IS HLA-A2?
HLA-A2 is part of a family of proteins that help your body’s immune system distinguish ‘self’ from ‘foreign’.
Not everyone has HLA-A2 in their body. A person is called HLA-A2 positive if they have the HLA-A2 protein in their body, and HLA-A2 negative if they do not have it.
Your doctor will be able to tell you if you are HLA-A2 positive or negative.
About kidney transplantation and TX200
Learn more about kidney transplantation and TX200 here
WHEN RECIPIENT AND DONOR ARE ‘HLA-A2 MISMATCHED’
If a kidney recipient is HLA-A2 negative and the donor is HLA-A2 positive, they are known as ‘HLA-A2 mismatched’. The immune system of the kidney recipient would recognise this mismatch and this can potentially lead to the recipient’s body rejecting the donor organ.
OVERVIEW OF THE STEADFAST STUDY
STEADFAST is a Phase I/II clinical study in an adult population (aged 18–70 years) with ESRD who are waiting to receive a new kidney from a living donor. The study is designed to test the safety and tolerability of the investigational product, TX200. Tolerability refers to the degree to which adverse effects of a drug can be tolerated by an individual.
WHAT ARE REGULATORY T CELLS?
Regulatory T cells, also called regulatory T lymphocytes or ‘Tregs’, are a type of specialised white blood cell which are important in helping the body’s immune system to function normally.
In transplantation settings, Tregs could take on the role of peacekeepers, directing other white blood cells to cease fire. This ensures the immune system does not mistakenly attack the healthy transplanted organ while still allowing the white blood cells to protect the body from harm, for instance from viruses and bacteria.
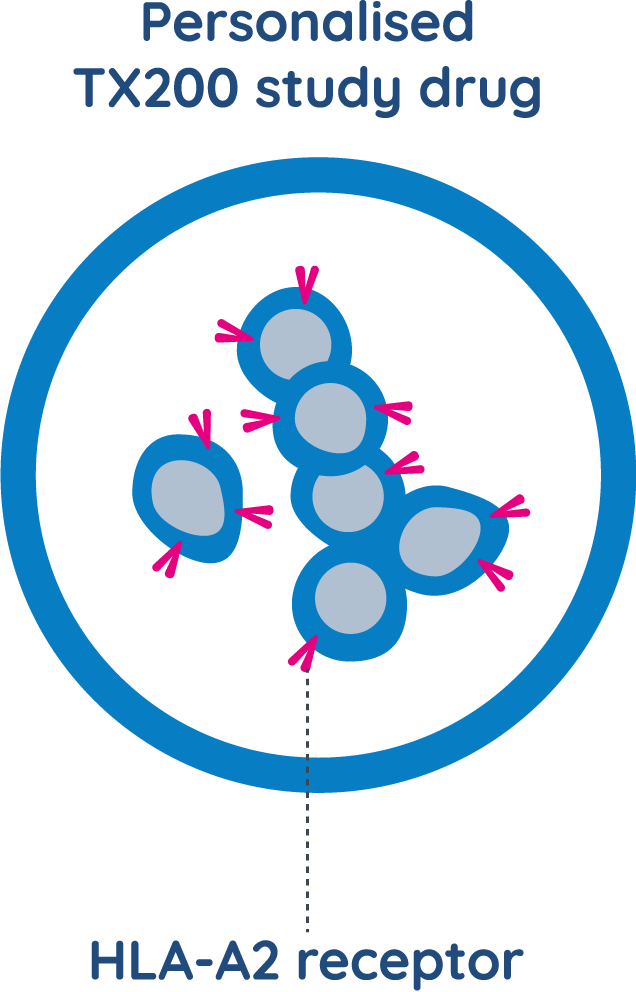
WHAT IS TX200 MADE FROM?
TX200 is an investigational cell therapy which is made from a study participant’s own regulatory T cells. The participant’s cells are collected before transplantation and genetically modified to recognise the HLA-A2 protein of the living organ donor.
Then, these personalised TX200 cells are administered back to the participant after their transplantation. TX200 is specifically designed for patients who are HLA-A2 negative and are expecting to receive a kidney from an HLA-A2 positive living donor.
TX200 is made using regulatory T cells, or Tregs, which are genetically modified to recognise the HLA-A2 protein on the transplanted kidney.
The goal of treatment with TX200 is to help the patient’s body accept the donor kidney, prevent the immune system from rejecting it, and allow it to function well for a long period of time.
It is currently not known if TX200 will help the acceptance of the donated kidney, or if there will be any side effects in humans, as this is the first time this study drug will be given to humans. It is also currently not known how long the effects of TX200 will last. The purpose of this clinical research study is to investigate the effects of TX200 in humans.
The study doctor will discuss the potential benefits and risks of taking part in the study with you in detail and will provide you with this information in writing.
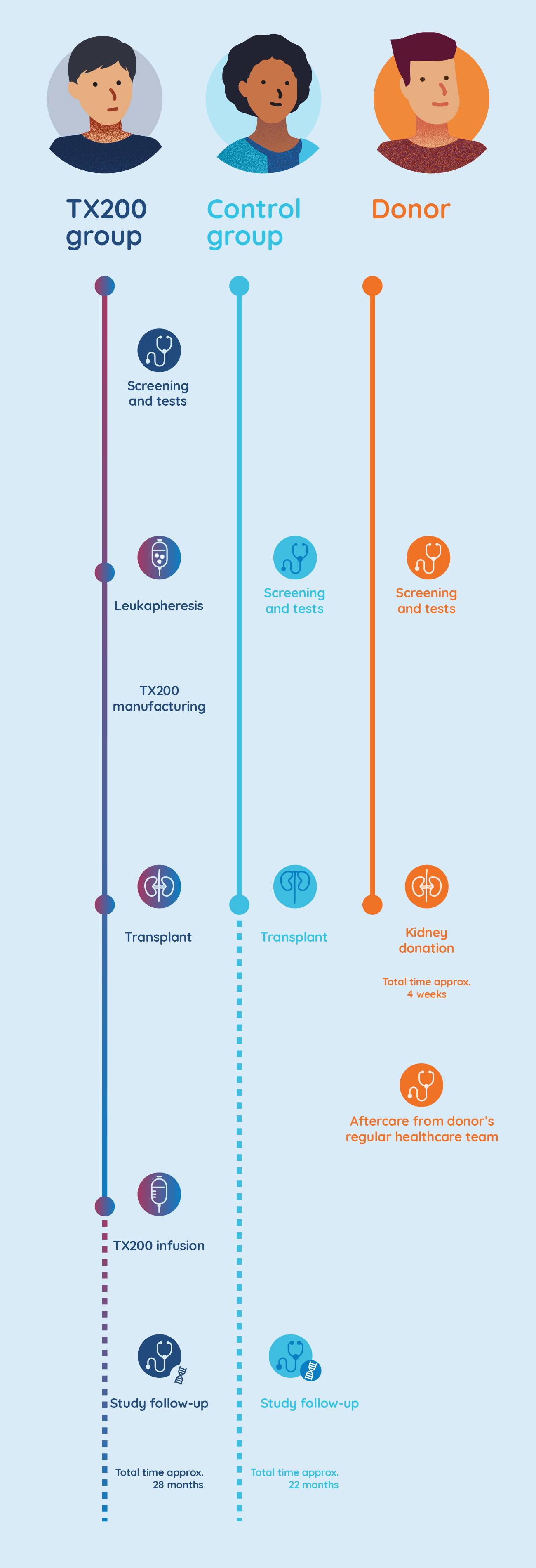
TX200 group
Using your own cells to make a TX200 study drug that is unique to you
There are two groups within this study: the ‘TX200’ participant group, will receive the study drug TX200 in addition to routine follow-up and current standard-of-care medications and the ‘control’ participant group, who will only receive routine follow-up and current standard-of-care medications.
Participants will be enrolled in either the TX200 group or in the control group. Before agreeing to participate in the study, they will know which group they are enrolled in.
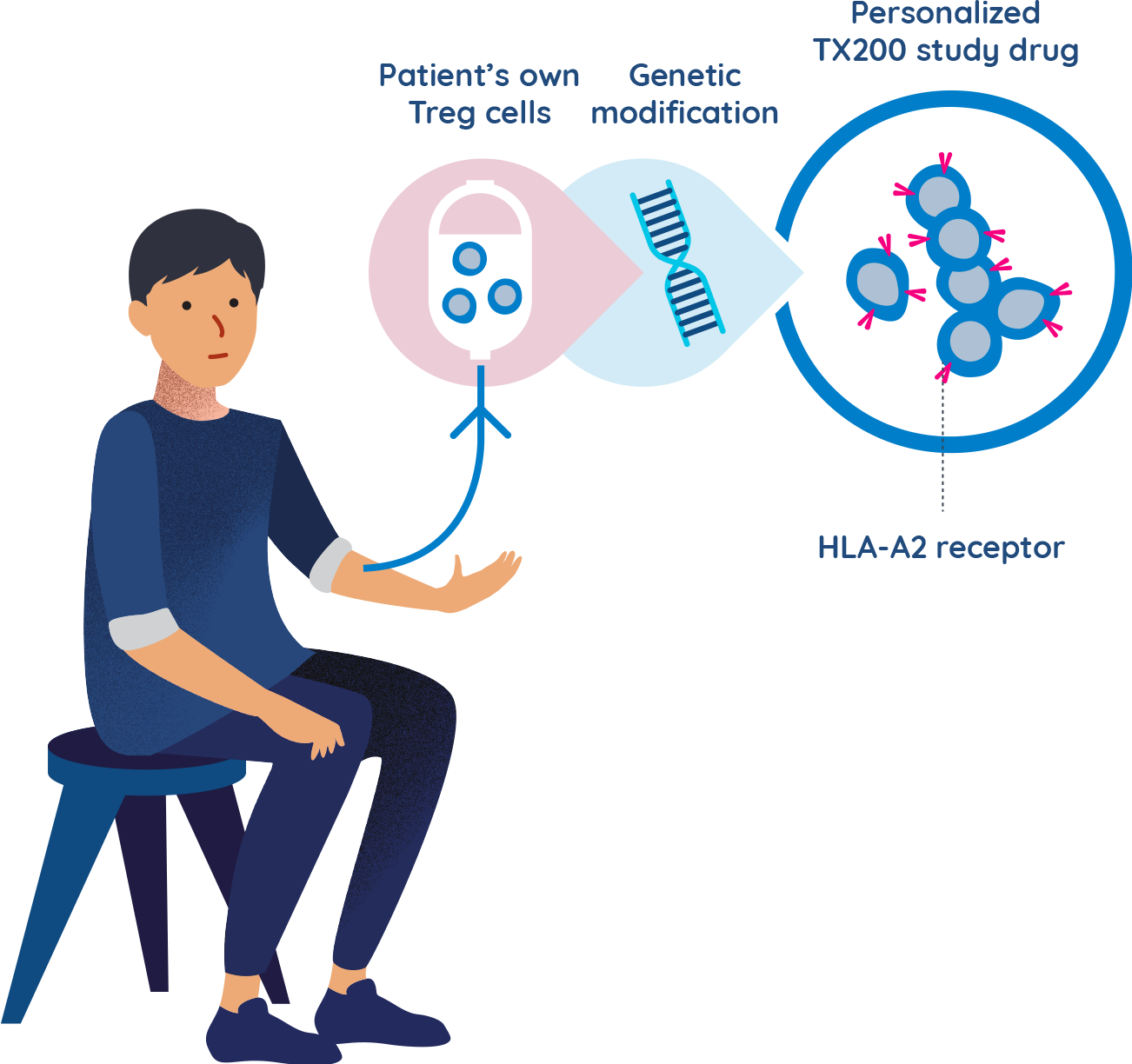
TX200 group participants undergo leukapheresis and their Treg cells are genetically modified
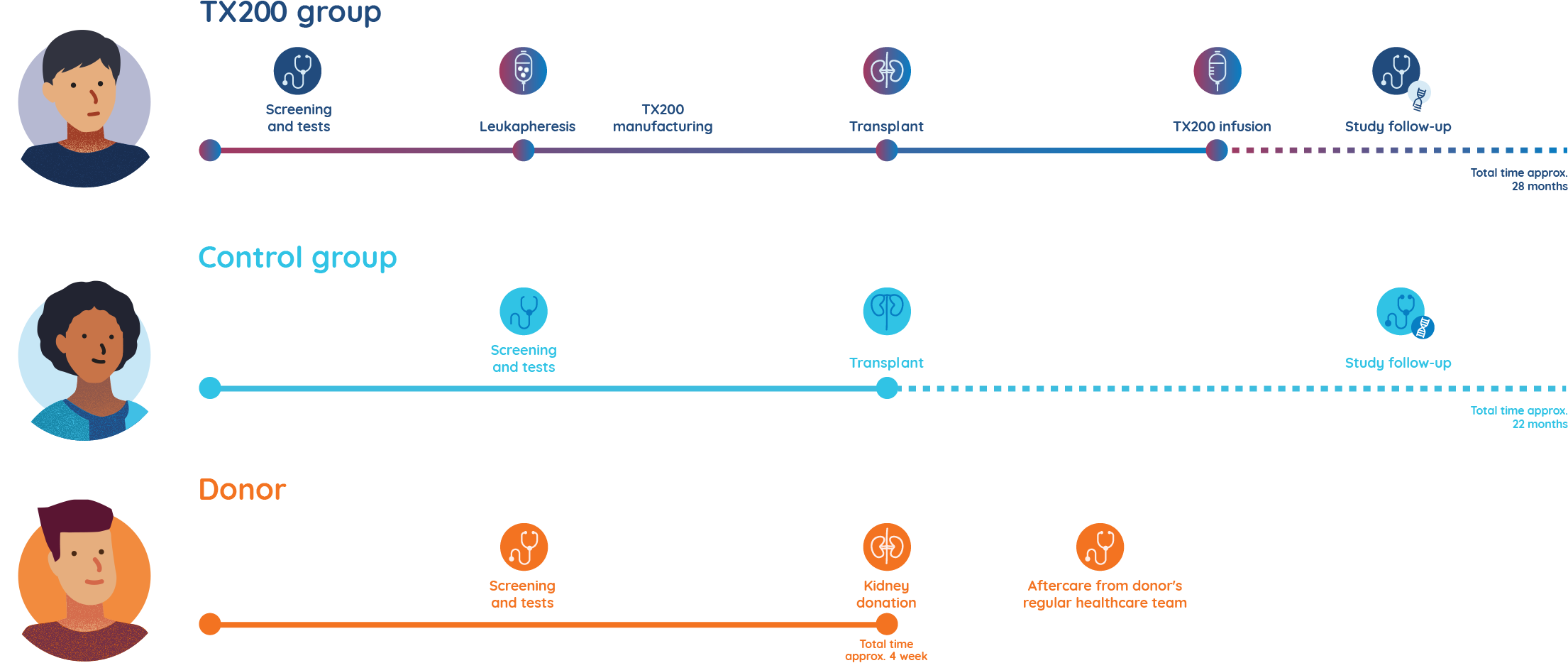
- A few weeks before the transplant surgery is scheduled, participants assigned to the TX200 group will undergo a procedure called leukapheresis at the hospital. Leukapheresis involves the collection of some of the participant’s blood cells. It takes approximately 3 to 5 hours to complete.
- The TX200 participant’s blood cells are then processed in a laboratory to separate out their specialist immune cells called regulatory T cells.
- The TX200 participant’s regulatory T cells are then genetically modified to recognise the HLA-A2 protein which will be present on the participant’s future transplanted kidney.
TX200 group participants receive the donated kidney
- The TX200 group participant will then receive the kidney from his/her living donor who is HLA-A2 positive. He or she will receive standard-of-care immunosuppressive medication starting the day of the transplant surgery.
TX200 group participants receive their personalised TX200 study drug
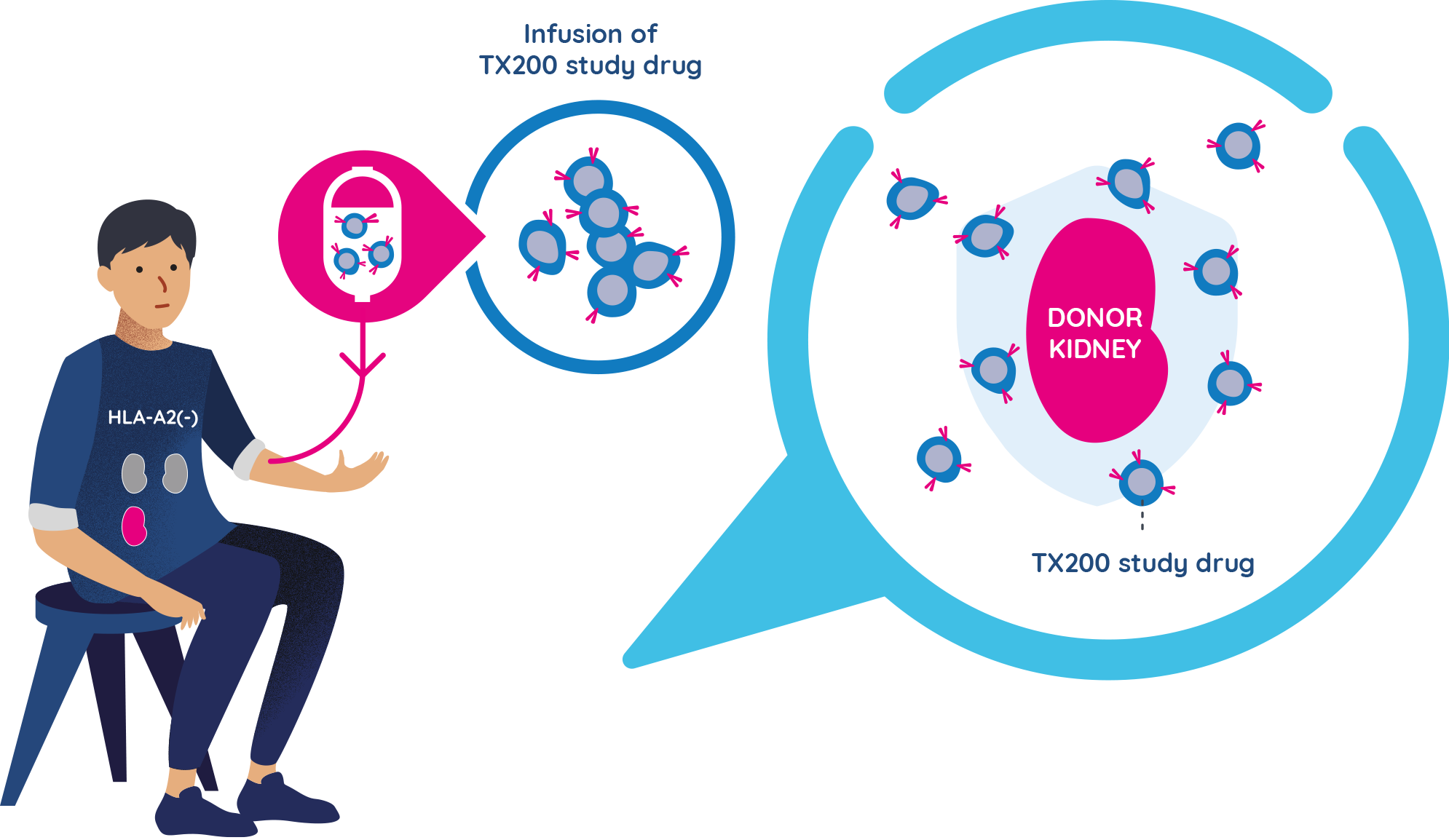
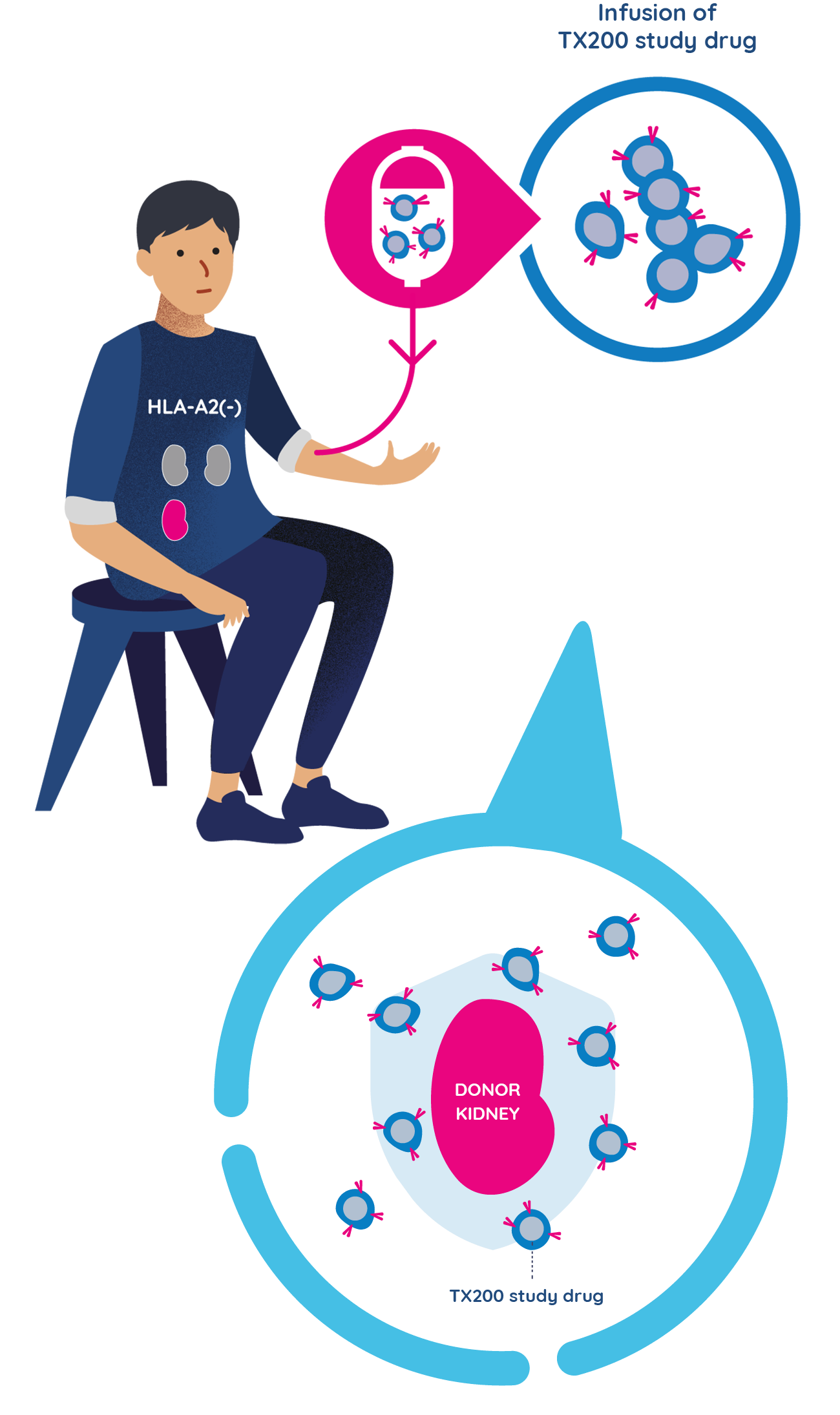
- Approximately three months after the TX200 group participants have received their donated kidney, they will receive their personalised TX200 study drug in the hospital, as an infusion into their blood.
- They will remain at the hospital for 24 to 48 hours after the TX200 infusion to be monitored closely. Each TX200 group participant will only have one infusion of TX200.
- Once infused, it is expected that many of the TX200 cells will find their way to the donated kidney, targeting the HLA-A2 protein. By attaching to the HLA-A2 protein on the donor kidney, it is expected to turn on the regulatory T cells that are in the TX200 study drug. Once they are turned on, the regulatory T cells should then help prevent any potential inflammation or immune response against the transplanted kidney.
- The study doctor will aim to progressively reduce the doses of the immunosuppression medications, to the lowest possible dose for each individual participant.
Why a ‘control’ participant group is important
It is important to evaluate the effect of the study drug alongside treatments currently used in routine practice. Therefore, some participants do not receive the study drug, but just continue with standard clinical care. They are in the control participant group.
The participants in the control group will also have a kidney transplant, with the same post-transplant care and follow-up immunosuppression medication, but they will not undergo leukapheresis or receive TX200.
Donors can help shape the future for kidney transplant recipients
In this study, the role of the organ donor is extremely important to our research, allowing us to assess the safety and the activity of TX200 in transplant recipients. It also helps researchers to understand the outcomes of the kidney transplant in participants receiving TX200 and in participants from the control group.
Donors will be closely monitored and will receive full supportive care for the period of their operation. They will also receive free access to any treatment that is related to their participation in the trial. Before, during and after the kidney transplant surgery, donors will continue to see their regular doctor and any specialists that normally manage their care.
Donors will be informed if their kidney is being transplanted into a participant in the TX200 group or in the control group. Regardless of whether the donated kidney recipient receives the study drug or not, the donor’s contribution will have a significant impact on the recipient’s life.
STEADFAST study stages
YOUR CHOICES
Medical advances depend on the participation of patients in clinical studies. Every participant in the STEADFAST study makes a positive contribution towards a better future for those individuals undergoing potentially life-saving organ transplantation.
Your participation in the study would be entirely voluntary. If you agree to participate, you can change your mind about being in the study at any time. Should you decide to withdraw from the study, it is important that your health continues to be monitored, in particular for TX200 group participants because this is the first time that TX200 has been administered in humans and the long-term effects are still unknown.
All kidney recipients (treated or not with TX200) who have completed the STEADFAST study will be invited to take part in a long-term follow-up study, to continue monitoring their health for another 13.5 years. Click here for more information
WHERE TO FIND FURTHER INFORMATION ABOUT THE STEADFAST STUDY
You may want to discuss this study with your doctor and with your family and friends to hear their views before making a final decision about participating.
If you need more information or would like to discuss the STEADFAST study further, the study team remains available at your convenience. The study doctor can discuss in detail the potential risks and benefits of taking part in the study, as well as provide you with written information.
Thank you for taking an interest in this study. Together, we hope that our genetically modified cell therapy, TX200 could help us to improve kidney transplant success.
ABOUT SANGAMO
Sangamo Therapeutics is a genomic medicine company with offices in the United States, England and France. We are committed to translating ground-breaking science into genomic medicines with the potential to transform patients’ lives using gene therapy, cell therapy, and genome engineering. For more information about Sangamo, visit www.sangamo.com.
ABOUT THE COVID-19 PANDEMIC
During the period of coronavirus pandemic, screening and testing for potential COVID-19 infection will be performed at certain time points during the study. In addition, some study visits may be performed remotely, in your home or over the telephone.
For more details on the study, visit EU Clinical Trials Register or clinicaltrials.gov and speak with the study team.
